Clinical Medical Guide for Tuberculosis
Clinical Medical Guide for Tuberculosis
COVID-19 Information for TB Programs
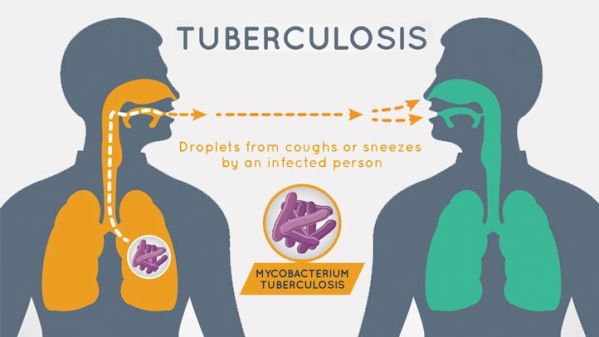
People with COVID-19 infection might seek care at tuberculosis (TB) clinics because the primary symptoms of this disease are similar to those of TB (e.g., fever, cough, and shortness of breath).
Patients with TB can become infected with COVID-19, which could result in an unexpected deterioration of a patient’s condition (e.g., was responding well to treatment and develops new acute cough, fever, or shortness of breath).
TB patients who are at least 65 years old; have respiratory compromise from their TB; or other medical conditions, including [HIV] and other immunocompromising conditions, are at greater risk for severe COVID-19 infection.
Therefore, respiratory [infection control] precautions, with which TB programs are familiar, are of even greater importance now as are general precautions, such as frequent hand washing, disinfecting surfaces, and avoiding touching one’s face.
COVID-19 guidance for healthcare facilities, including outpatient facilities, is available at the CDC COVID-19 [website] .

The Importance of TB Treatment
It is very important that people who have tuberculosis (TB) disease are treated, take the drugs exactly as prescribed, and finish the medicine.
If they stop taking the drugs too soon, they can become sick again.
If they do not take the drugs correctly, the TB bacteria that are still alive may become resistant to those drugs.
TB that is resistant to drugs is harder and more expensive to treat.
On This Page
* [COVID-19 Information for TB Programs]
* [COVID-19 Information for TB Laboratories]
Tuberculosis Information for Public Health Emergencies
A public health emergency can make efforts to help TB patients continue their treatment difficult.
TB programs’ work ensures that people who are receiving treatment for TB disease can continue their treatment, even if routine health care services are affected.
In the event of a public health emergency, TB programs can help to mitigate potential shifts in staff assignments and resources.
* Make sure contact information for patients is current, and staff can reach patients with updates and information.
* Provide contact numbers of TB program personnel to patients.
* Communicate any changes or updates in service to community partners.
* Consider providing additional medications to patients if health department operations are likely to be affected.
* If policies and procedures are in place, consider alternative treatment delivery methods, such as [electronic directly observed therapy]
The [National TB Molecular Surveillance Center] at the Michigan Department of Health and Human Services Bureau of Laboratories is operational, and referral of *M. tuberculosis*isolates should continue.
CDC is continuing to provide conventional genotyping results and conducting analysis of whole genome sequencing data, as applicable. For additional information, please contact Angie Schooley at schooleya@michigan.gov or 517-335-9637.
The response to COVID-19 is an unprecedented event and could impact public health laboratory testing for TB.
CDC has received reports of shifting or rotating mycobacteriology staff to support COVID-19 testing and also has received notification that some public health laboratories are implementing continuity of operations plans.
As always, CDC encourages regular communication between TB programs and public health laboratories to understand any impacts to TB testing capacity.
 Join/ Renew
Join/ Renew